Adrulipase is a recombinant lipase enzyme for the treatment of exocrine pancreatic insufficiency (EPI) associated with cystic fibrosis (CF) and chronic pancreatitis (CP). Adrulipase, supplied as an oral, non-systemic, biologic capsule, is derived from the Yarrowia lipolytica yeast lipase and breaks up fat molecules in the digestive tract of EPI patients so that they can be absorbed as nutrients.
EPI is a condition characterized by deficiency of the exocrine pancreatic enzymes, resulting in a patient’s inability to digest food properly, or maldigestion. The deficiency in this enzyme can be responsible for greasy diarrhea, fecal urge and weight loss.
The digestive standard of care for both CF and chronic pancreatitis (CP) patients with EPI are commercially-available PERTs. Ideally, a stable daily dose of PERT will enable CF patients to eat a normal to high-fat diet and minimize unpleasant gastrointestinal symptoms. In practice, however, a substantial number of CF patients do not achieve normal absorption of fat with PERTs. Moreover, PERTs require the administration of as many as 40 pills per day and have potential issues with Black Box safety warnings.
In developing adrulipase, Entero Therapeutics is seeking to provide CF and CP patients with a safe and effective therapy to control EPI that is non-animal derived and offers the potential to dramatically reduce their daily pill burden.
Capeserod is a selective 5-HT4 receptor partial agonist, which Entero Therapeutics licensed from Sanofi and is repurposing and developing for GI indications. Sanofi previously conducted seven Phase 1 and two Phase 2 trials investigating the potential of capeserod for neurological disorders. In these trials, involving over 600 patients, Capeserod appeared safe and well-tolerated.
Sanofi’s research on capeserod determined the drug’s mechanism of action may directly or indirectly initiate the peristaltic or secretory reflex by releasing neurotransmitters that can decrease colonic transit time and improve bowel movement in constipation. Additionally, capeserod may reduce the likelihood of inflammation and damage of epithelial cells where its partial agonistic effect is expected via stimulation of 5HT4b in intestinal epithelium. Moreover, combination therapy with ADBR3 agonists may provide additive effect and relief in symptoms in ulcerative colitis.
Based on this research and subsequent artificial intelligence (AI)-empowered analyses, Entero Therapeutics believes capeserod has potential applications for several gastrointestinal disorders in multibillion-dollar markets where there are significant unmet clinical needs. These GI indications include gastroparesis, a disease whereby the movement of food from the stomach to the small intestine is delayed, resulting in severe constipation, and pediatric ulcerative colitis, an inflammatory bowel disease (IBD) that causes irritation, inflammation, and ulcers in the lining of the colon.
Latiglutenase is an orally administered mixture of two gluten-specific recombinant proteases that degrades gluten proteins into small physiologically irrelevant fragments that is designed to be administered as an adjunct to a gluten-free diet (GFD).
In Phase 2a and 2b clinical trials, latiglutenase has been shown to mitigate gluten-induced intestinal mucosal injury as well reduce the severity and frequency of symptoms in celiac disease patients. Evidence of symptom relief was particularly pronounced for patients who continue to have positive serology to gluten-induced antibodies (seropositive) despite following a GFD as seen in the results shown below (Syage 2017, 2019). These studies also demonstrated that Latiglutenase is well tolerated by CD patients with no discernable difference in adverse event profile for active vs. placebo treated patients.
Figure 1: Percent reductions in symptom severity relative to placebo for the combined 600 mg and 900 mg treatment groups.
Additionally, in the CeliacShield™ trial (NCT03585478) conducted at Mayo Clinic (Murray 2022), the gluten-challenge trial measured several outcomes including histology, symptoms, serology, and gluten in urine. Histologic protection was assessed by measuring changes in villous height to crypt depth ratio (ΔVh:Cd) and intraepithelial lymphocytes (ΔIEL) before and after a 6-week, 2-g per day gluten challenge period. The attenuation of ΔVh:Cd and ΔIEL for the active (1200-mg latiglutenase) group relative to placebo was 88% and 60% with p-values of 0.0570 and 0.0181 (ANCOVA), respectively.
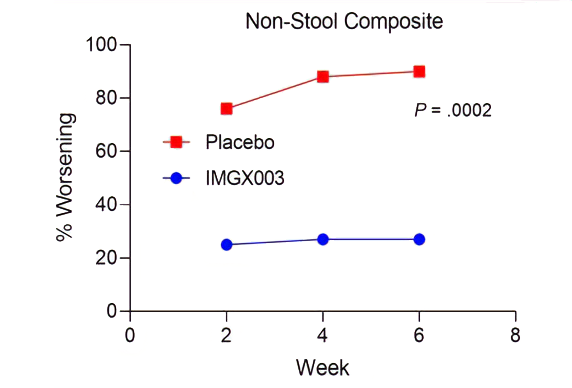
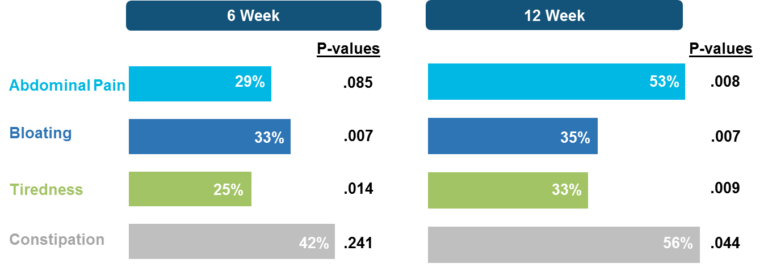
Measurements of gluten immunogenic peptides (GIP) in urine showed reduction of gluten of about 95% for latiglutenase vs. placebo with p < 0.0001 (Figure 2) demonstrating the mechanism of action. The reduction in symptom severity for latiglutenase relative to placebo ranged from 53% to 99% for abdominal pain, bloating, tiredness and the overall non-stool composite scale that also includes nausea. All of these symptoms showed consistent trends when monitored over three 2-week intervals with p values ranging from <0.001 to 0.030 (see Figure 3).
Figure 2. GIP concentration in urine before, during and after the gluten challenge treatment period
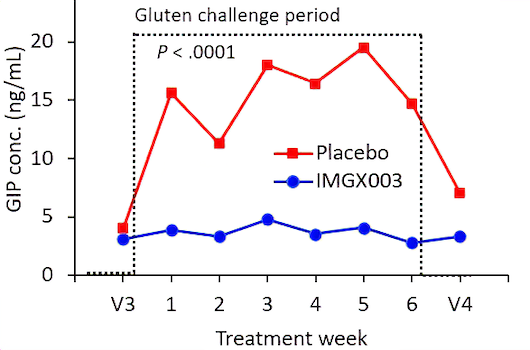
Figure 3. Mean change from baseline (% worsening) for non-stool composite for three 2-week gluten challenge treatment periods for latiglutenase vs. placebo.